- Joined
- Jul 25, 2008
- Messages
- 1,700
The secret is this thread is about so much more then the title
This thread is now out of date. We have moved into 3rd generation peptides & greatly advanced our knowledge.
 [/CENTER]
[/CENTER]
I am not a doctor of medicine and everything discussed herein is meant solely to share science and explore ideas and really cool stuff that nobody gives a damn about sharing with those beyond the cloister. - DatBtrue
Latest News
Introduction to the thread
What is growth hormone?
Synthetic Growth Hormone is an artificially created hormone "identical" to the major naturally produced (endogenous) isoform. It is often referred to by its molecular mass which is 22kDa (kilodaltons) and is made up of a sequence of 191 amino acids (primary structure) with a very specific folding pattern that comprise a three-dimensional structure (tertiary structure). This tertiary structure is subject to potential shape change through a process known as thermal denaturation. While many labs are capable of generating growth hormone (GH) with the proper primary structure not all will be capable of creating a tertiary structure identical to the major naturally occurring growth hormone. The tertiary structure can determine the strength with which the growth hormone molecule binds to a receptor which will in turn affect the "strength" of the intracellular signaling which mediates the events leading to protein transcription, metabolism, IGF-1 creation, etc. It is this inconsistency that accounts in part for the differences in effectiveness of various non-pharmaceutically produced synthetic growth hormone.
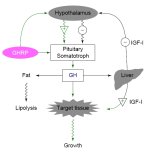
Naturally produced Growth Hormone is produced in the anterior pituitary and to a far lesser extent in peripheral tissue. It is made up of a blend of isoforms the majority of which is the 22kDa (191 amino acid) variety with which most are familiar. In addition an isoform that is missing the 15 amino acids that interact with the prolactin receptor is also produced. This form is known as 20kDa and although it binds differently to the growth hormone receptor it has been shown to be equally potent to 22kDa. It appears that 20kDa has lower diabetogenic activity then 22kDa. The pituitary releases a blend of these two isoforms with 20kDa averaging perhaps 10% of the total although this percentage increases post-exercise. Currently there is no synthetic produced for external administration for this isoform.
Growth hormone (GH) in the body is released in pulsatile fashion. It has been demonstrated that this pattern promotes growth. The pituitary is capable of rather quickly synthesizing very large amounts of growth hormone which it stores large amounts in both a finished and unfinished form. Adults rarely experience GH pulses (i.e. releases of pituitary stores) that completely deplete these stores. As we age we do not lose the ability to create and store large amounts of growth hormone. Rather we experience a diminished capacity to "instruct" their release. The volume of GH that is released can not be properly equated to the exogenous administration of synthetic GH for the reason that a set of behavioral characteristics accompany natural GH that differ from those of synthetic GH. Among those characteristics are concentrated pulsatile release which upon binding in mass to growth hormone receptors on the surface of cells initiate signaling cascades which mediate growth events by translocating signaling proteins to the nucleus of the cell where protein transcription and metabolic events occur.
These very important signaling pathways desensitize to Growth Hormone's initiating effects and need to experience an absence of Growth Hormone in order to reset and be ready to act again. The presence of GH released in pulsatile fashion is graphed as a wave with the low or no growth hormone period graphed as a trough. Therefore attempting to find a natural GH to synthetic GH equivalency is not very productive because in the end what is probably import is:
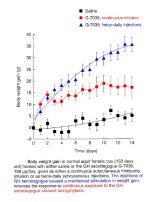
Synthetic GH versus Natural GH in IUs
An attempt has been made on my part and can be found at:
Rather than demonstrate absolute values this comparison articles should serve to demonstrate that the body can produce pharmacological levels of growth hormone.
Brief overview of natural GH release
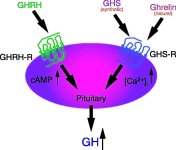
The initiation of growth hormone release in the pituitary is dependent on a trilogy of hormones:
In the aging adult these Ghrelin-mimetics or the GHRPs restore a more youthful ability to release GH from the pituitary as they turn down somatostatin's negative influence which becomes stronger as we age and turn up growth hormone releasing hormone's influence which becomes weaker as we age.
The exogenous administration of Growth Hormone Releasing Hormone (GHRH) creates a pulse of GH release which will be small if administered during a natural GH trough and higher if administered during a rising natural GH wave.
Growth Hormone Releasing Peptides (GHRP-6, GHRP-2, Hexarelin) are capable of creating a larger pulse of GH on their own then GHRH and they do this with much more consistency and predictability without regard to whether a natural wave or trough of GH is currently taking place.
Synergy of GHRH + GHRP
It is well documented and established that the concurrent administration of Growth Hormone Releasing Hormone (GHRH) and a Growth Hormone Releasing Peptide (GHRP-6, GHRP-2 or Hexarelin) results in synergistic release of GH from pituitary stores. In other words if GHRH contributes a GH amount quantified as the number 2 and GHRPs contributed a GH amount quantified as the number 4 the total GH release is not additive (i.e. 2 + 4 = 6). Rather the whole is greater than the sum of the parts such that 2 + 4 = 10.
While the GHRPs (GHRP-6, GHRP-2 and Hexarelin) come in only one half-life form and are capable of generating a GH pulse that lasts a couple of hours re-administration of a GHRP is required to effect additional pulses.
Growth Hormone Releasing Hormone (GHRH) however is currently available in several forms which vary only by their half-lives. Naturally occurring GHRH is either a 40 or 44 amino acid peptide with the bioactive portion residing in the first 29 amino acids. This shortened peptide identical in behavior and half-life to that of GHRH is called Growth Hormone Releasing Factor and is abbreviated as GRF(1-29).
GRF(1-29) is produced and sold as a drug called Sermorelin. It has a short-half life measured in minutes. If you prefer analogies think of this as a Testosterone Suspension (i.e. unestered).
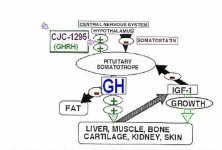
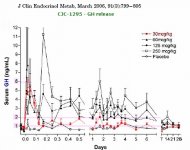
To increase the stability and half-life of GRF(1-29) four amino acid changes where made to its structure. These changes increase the half-life beyond 30 minutes which is more than sufficient to exert a sustained effect which will maximize a GH pulse. This form is often called tetrasubstituted GRF(1-29) (or modified) and unfortunately & confusingly mislabeled as CJC-1295. If you prefer analogies think of this as a Testosterone Propionate (i.e. short-estered).
Note that some may also refer to this as CJC-1295 without the DAC (Drug Affinity Complex).
Frequent dosing of either the aforementioned modified GRF(1-29) or regular GRF(1-29) is required and as previously indicated works synergistically with a GHRP.
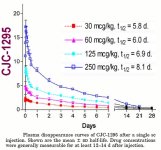
In an attempt to create a more convenient long-lasting GHRH, a compound known as CJC-1295 was created. This compound is identical to the aforementioned modified GRF(1-29) with the addition of the amino acid Lysine which links to a non-peptide molecule known as a "Drug Affinity Complex (DAC)". This complex allows GRF(1-29) to bind to albumin post-injection in plasma and extends its half-life to that of days. If you prefer analogies think of this as a Testosterone Cypionate (i.e. long-estered). However this is not accurate. CJC-1295 results in continual GH bleed. Although natural pulsation still occurs CJC-1295 does nothing to increase those pulses. Instead it raises base levels of GH and creates a more feminized pattern of release. This not desirable.
Modified GRF(1-29)however when combined with a GHRP brings about a substantial pulse which has desirable effects.
What follows on this first page of the thread is:
If all of this is a bit unclear because a lot of new concepts are thrown at you one of my original very thorough articles is available:
I have only one pet-peeve and that is when someone refers to synthetic growth hormone as "real" growth hormone. The GH that your body produces is as real as it gets. It is what grew you from a fetus to a boy (girl) and from a boy (girl) to a man (woman). - DatBtrue
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Peptides (proteins) are present in every living cell and possess a variety of biochemical activities. Some peptides are synthesized in the ribosomes of a cell by translation of mRNA (messenger RNA) into hormones and signaling molecules for example. Other peptides are assembled (rather then synthesized) and become enzymes with a vast variety of functions. Peptides also make up the structure of receptors which await binding of hormones & signaling molecules.
A peptide is a molecule created by joining two or more amino acids. In general if the number of amino acids is less than fifty, these molecules are called peptides, while larger sequences are referred to as proteins.
So peptides can be thought of as tiny proteins. They are merely strings of amino acids.
Raw Constituents of Peptides (Amino Acids)
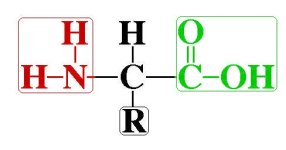
Amino acids are small molecules made up of atoms. As part of their structure they posses a grouping of a Nitrogen (N) atom bonded to two Hydrogen (H) atoms. This is called an amino group and written as (NH2). In addition their structure is also made up of a second grouping of a Carbon (C) atom bonded to two Oxygen (O) and one Hydrogen atom. This group is called a carboxyl group and is written as (COOH).
Between these two groupings are atoms and bonds unique to each amino acid. In other words all amino acids possess the two groupings (amino & carboxyl) as end-points between which are sandwiched a unique set of atoms.
Amino Acids
Inside the human body there are twenty standard amino acids used by cells in peptide biosynthesis (i.e. the cellular creation of peptides from amino acids). Our genetic code specifies how to synthesize peptides and proteins from these amino acids.
Amino acids are classified into two groups: essential amino acids and nonessential amino acids.
An essential amino acid is an indispensable amino acid which cannot be made by the body and must be supplied by food. These include isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine. Another amino acid - histidine is considered semi-essential because the body does not always require dietary sources of it.
Nonessential amino acids are made by the body from the essential amino acids or the routine breakdown of proteins. The nonessential amino acids are arginine, alanine, asparagine, aspartic acid, cysteine, glutamine, glutamic acid, glycine, proline, serine, and tyrosine.
All twenty amino acids are equally important in maintaining a healthy body. They are the raw constituents of peptides and proteins.
The standard abbreviations for amino acids come in two forms: a one letter form and a three letter form. They are:
A - Ala - Alanine
C - Cys - Cysteine
D - Asp - Aspartic Acid
E - Glu - Glutamic Acid
F - Phe - Phenylalanine
G - Gly - Glycine
H - His - Histidine
I - Ile - Isoleucine
K - Lys - Lysine
L - Leu - Leucine
M - Met - Methionine
N - Asn - Asparagine
P - Pro - Proline
Q - Gln - Glutamine
R - Arg - Arginine
S - Ser - Serine
T - Thr - Threonine
V - Val - Valine
W - Trp - Tryptophan
Y - Tyr - Tyrosine
Amino acids exist in either D (dextro) or L (levo) form. Most of the amino acids found in nature (and all within human cells) are of the L-form. As a generality all amino acids except glycine have a mirror image of the L-form. This mirror image is called the D-form. It is common when referring to the L-form (naturally occurring form) to leave off the "L" designation whereas the "D" designation is always explicitly written.
D-amino acids are found naturally in bacterial cell walls and used in some synthetic peptides to make a peptide more stable or more resistant to degradation.
Amino Acid + Amino Acid = Peptide
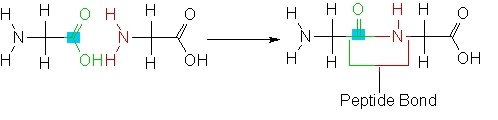
The amino acids are joined together by what is known as a "peptide bond". A "peptide bond" is a linkage in which the nitrogen atom of one amino acid (from the amino group (NH2) binds to the carbon atom of another amino acid's carboxyl group (COOH).
During this binding process a molecule of water is released. This is called a condensation reaction.
The resulting CO-NH bond is called a peptide bond, and the resulting molecule is called an amide.
On the following image note that the COOH group gives up an Oxygen Hydrogen (OH) bond and the NH2 group gives up a Hydrogen (H). This forms H2O, which is a water molecule which is not part of the newly created peptide. NOTE: in the following image the C (carbon) symbol is missing as it is assumed so I indicate it with a blue square.
This reaction creating a peptide bond between two amino acids creates a peptide. We can call this peptide (made up of two amino acids) a dipeptide.
This process can be repeated using the twenty amino acids as raw material to create longer peptide chains. Sometimes peptide chains consisting of fifty to 100 amino acids are called polypeptides. Often a peptide chain beyond 100 amino acids is called a protein.
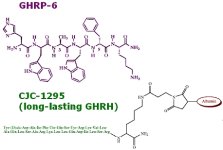
GHRP-6 is a peptide made up of just six amino acids. It's structure is often written as His-DTrp-Ala-Trp-DPhe-Lys-NH2
Note that the Carboxyl grouping (COOH) is assumed in the first position and is usually not written. The amino group (NH2) is wrtitten in the last position. The "meat" or the part that makes GHRP-6 distinct is the seqence in the middle of histadine bonded to the "D" form of Tryptophan bonded to Alanine bonded to Tryptophan bonded to the "D" form of Phenylalanine bonded to Lysine.
Pepdide bonds are formed by water (H2O) condensation (removing water). The converse is also true. A peptide bond can be broken down by hydrolysis (adding water).
The Amino Acid Structures of Peptides discussed in this thread
Growth Hormone Releasing peptides (GHRPs) (GH pulse initiators):
Growth Hormone Releasing Hormone (GHRH) (amplifies the GHRP initiated pulse):
The analog in the above quoted study resisted degradation for 30 minutes. The quote implies that if your analog can last 30 minutes it has tapped out the potential for a single pulse.
Since another pulse won't be generated for about 2.5 - 3 hours analogs that last more than 30 minutes up to 3 hours are not any more beneficial.
You would need an analog that kept growth hormone releasing hormone around beyond 3 hours to have it trigger a second pulse.
Otherwise dosing the 30 minute analog every 3 hours will maximize GH output OR you could just use an analog such as CJC-1295 which lasts for many days and will trigger several GH pulses a day for several days on a single dose.
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
A Brief Summary of Dosing and Administration
Dosing GHRPs
The saturation dose in most studies on the GHRPs (GHRP-6, GHRP-2, Ipamorelin & Hexarelin) is defined as either 100mcg or 1mcg/kg.
What that means is that 100mcg will saturate the receptors fully, but if you add another 100mcg to that dose only 50% of that portion will be effective. If you add an additional 100mcg to that dose only about 25% will be effective. Perhaps a final 100mcg might add a little something to GH release but that is it.
So 100mcg is the saturation dose and you could add more up to 300 to 400mcg and get a little more effect.
A 500mcg dose will not be more effective then a 400mcg, perhaps not even more effective then 300mcg.
The additional problems are desensitization & cortisol/prolactin side-effects.
Desensitization
Chronic use of GHRP-6 at 100mcg dosed several times a day every day will not cause pituitary problems, nor significant prolactin or cortisol problems, nor desensitize.
GHRH
Now Sermorelin, GHRH (1-44) and GRF(1-29) all are basically GHRH and have a short half-life in plasma because of quick cleavage between the 2nd & 3rd amino acid. This is no worry naturally because this hormone is secreted from the hypothalamus and travels a short distance to the underlying anterior pituitary and is not really subject to enzymatic cleavage. The release from the hypothalamus and binding to somatotrophs (pituitary cells) happens quickly.
However when injected into the body it must circulate before finding its way to the pituitary and so within 3 minutes it is already being degraded.
That is why GHRH in the above forms must be dosed high to get an effect.
GHRH analogs
All GHRH analogs swap Alanine at the 2nd position for D-Alanine which makes the peptide resistant to quick cleavage at that position. This means analogs will be more effective when injected at smaller dosing.
The analog tetra or 4 substituted GRF(1-29) sometimes called CJC w/o the DAC or referred to by me as modified GRF(1-29) has other amino acid modifications. They are a glutamine (Gln or Q) at the 8-position, alanine (Ala or A) at the 15-position, and a leucine (Leu or L) at the 27-position.
The alanine at the 8th position enhances bioavailability but the other two amino substitutions are made to enhance the manufacturing process (i.e. create manufacturing stability).
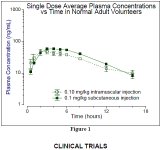
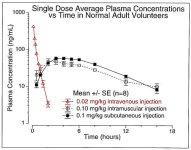
For use in vivo, in humans, the GHRH analog known as CJC w/o the DAC or tetra (4) substituted GRF(1-29) or modified GRF(1-29) is a very effective peptide with a half-life probably 30+ minutes.
That is long enough to be completely effective.
The saturation dose is also defined as 100mcg.
Problem w/ Using any GHRH alone
The problem with using a GHRH even the stronger analogs is that they are only highly effective when somatostatin is low (the GH inhibiting hormone). So if you unluckily administer in a trough (or when a GH pulse is not naturally occurring) you will add very little GH release. If however you luckily administer during a rising wave or GH pulse (somatostatin will not be active at this point) you will add to GH release.
Solution is GHRP + GHRH analog
The solution is simple and highly effective. You administer a GHRH analog with a GHRP. The GHRP creates a pulse of GH. It does this through several mechanisms. One mechanism is the reduction of somatostatin release from the hypothalamus, another is a reduction of somatostatin influence at the pituitary, still another is increased release of GHRH from the brain and finally GHRPs act on the same pituitary cells (somatotrophs) as do GHRHs but use a different mechanism to increase cAMP formation which will further cause GH release from somatotroph stores.
GHRH also has a way of reciprocally reinforcing GHRPs action.
The result is a synergistic GH release.
The GH is not additive it is synergistic. By that I mean:
A solid protocol
A solid protocol would be to use a GHRP + a GHRH analog pre-bed (to support the nightime pulse) and once or twice throughout the day.
For anti-aging, deep restful restorative sleep, the once at night dosing is all you need. For an adult aged 40+ it is enough to restore GH to youthful levels.
However for bodybuilding or fatloss or injury repair multiple dosings can be effective.
The GHRH analog can be used at 100mcg and as high as you want without problems.
The GHRP-6 can always be used at 100mcg w/o problems but a dose of 200mcg will probably be fine as well.
Again desensitization is something to keep an eye on particularly with the highest doses of GHRP-2 and all doses of Hexarelin.
This may be dosed several times a day to be highly effective.
When dosing multiple times a day at least 3 hours should separate the administrations.
The difference is once a day dosing pre-bed will give a youthful restorative amount of GH while multiple dosing and or higher levels will give higher GH & IGF-1 levels when coupled with diet & exercise will lead to muscle gain & fatloss.
Dose w/o food
Administration should ideally be done on either an empty stomach or with only protein in the stomach. Fats & carbs blunt GH release. So administer the peptides and wait about 20 minutes (no more then 30 but no less then 15 minutes) to eat. AT that point the GH pulse has about hit the peak and you can eat what you want.
This thread is now out of date. We have moved into 3rd generation peptides & greatly advanced our knowledge.
 [/CENTER]
[/CENTER]I am not a doctor of medicine and everything discussed herein is meant solely to share science and explore ideas and really cool stuff that nobody gives a damn about sharing with those beyond the cloister. - DatBtrue
Latest News
- 6/18/09 - The GH comparison article (post #8 & #9) has been lengthened to take a look at IGF-1
- 5/09/09 - Introduction, Basic Peptide Primer & A Brief Summary of usage has been added in post #1.
- 5/07/09 - The GH comparison article (post #8 & #9) has been completely rewritten.
- 4/22/09 - We are discovering that synthetic GH can be used together with GHRP/GHRH in a protocol
- 5/09/09 - Thread is slowly being indexed a rough draft can be found below
Introduction to the thread
What is growth hormone?
Synthetic Growth Hormone is an artificially created hormone "identical" to the major naturally produced (endogenous) isoform. It is often referred to by its molecular mass which is 22kDa (kilodaltons) and is made up of a sequence of 191 amino acids (primary structure) with a very specific folding pattern that comprise a three-dimensional structure (tertiary structure). This tertiary structure is subject to potential shape change through a process known as thermal denaturation. While many labs are capable of generating growth hormone (GH) with the proper primary structure not all will be capable of creating a tertiary structure identical to the major naturally occurring growth hormone. The tertiary structure can determine the strength with which the growth hormone molecule binds to a receptor which will in turn affect the "strength" of the intracellular signaling which mediates the events leading to protein transcription, metabolism, IGF-1 creation, etc. It is this inconsistency that accounts in part for the differences in effectiveness of various non-pharmaceutically produced synthetic growth hormone.
Naturally produced Growth Hormone is produced in the anterior pituitary and to a far lesser extent in peripheral tissue. It is made up of a blend of isoforms the majority of which is the 22kDa (191 amino acid) variety with which most are familiar. In addition an isoform that is missing the 15 amino acids that interact with the prolactin receptor is also produced. This form is known as 20kDa and although it binds differently to the growth hormone receptor it has been shown to be equally potent to 22kDa. It appears that 20kDa has lower diabetogenic activity then 22kDa. The pituitary releases a blend of these two isoforms with 20kDa averaging perhaps 10% of the total although this percentage increases post-exercise. Currently there is no synthetic produced for external administration for this isoform.
Growth hormone (GH) in the body is released in pulsatile fashion. It has been demonstrated that this pattern promotes growth. The pituitary is capable of rather quickly synthesizing very large amounts of growth hormone which it stores large amounts in both a finished and unfinished form. Adults rarely experience GH pulses (i.e. releases of pituitary stores) that completely deplete these stores. As we age we do not lose the ability to create and store large amounts of growth hormone. Rather we experience a diminished capacity to "instruct" their release. The volume of GH that is released can not be properly equated to the exogenous administration of synthetic GH for the reason that a set of behavioral characteristics accompany natural GH that differ from those of synthetic GH. Among those characteristics are concentrated pulsatile release which upon binding in mass to growth hormone receptors on the surface of cells initiate signaling cascades which mediate growth events by translocating signaling proteins to the nucleus of the cell where protein transcription and metabolic events occur.
These very important signaling pathways desensitize to Growth Hormone's initiating effects and need to experience an absence of Growth Hormone in order to reset and be ready to act again. The presence of GH released in pulsatile fashion is graphed as a wave with the low or no growth hormone period graphed as a trough. Therefore attempting to find a natural GH to synthetic GH equivalency is not very productive because in the end what is probably import is:
- the quantity & quality of intracellular signaling events; and
- the degree to which GH stimulates autocrine/paracrine (locally produced/locally used) muscle IGF-1 & post-exercise its splice variant MGF.
- the degree to which GH stimulates autocrine/paracrine (locally produced/locally used) muscle IGF-1 & post-exercise its splice variant MGF.
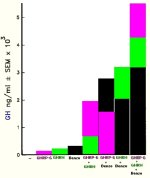
Synthetic GH versus Natural GH in IUs
An attempt has been made on my part and can be found at:
#8 - Growth Hormone Administration vs. CJC-1295/GHRP-6 + GHRH (part I of II)
#9 - Growth Hormone Administration vs. CJC-1295/GHRP-6 + GHRH (part II of II)
#9 - Growth Hormone Administration vs. CJC-1295/GHRP-6 + GHRH (part II of II)
Rather than demonstrate absolute values this comparison articles should serve to demonstrate that the body can produce pharmacological levels of growth hormone.
Brief overview of natural GH release
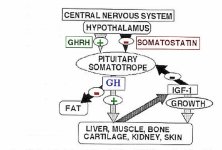
The initiation of growth hormone release in the pituitary is dependent on a trilogy of hormones:
Somatostatin which is the inhibitory hormone and responsible in large part for the creation of pulsation;
Growth Hormone Releasing Hormone (GHRH) which is the stimulatory hormone responsible for initiating GH release; and
Ghrelin which is a modulating hormone and in essence optimizes the balance between the "on" hormone & the "off" hormone. Before Ghrelin was discovered the synthetic growth hormone releasing peptides (GHRPs) were created and are superior to Ghrelin in that they do not share Ghrelin's lipogenic behavior. These GHRPs are GHRP-6, GHRP-2, Hexarelin and later Ipamorelin all of which behave in similar fashion.
Growth Hormone Releasing Hormone (GHRH) which is the stimulatory hormone responsible for initiating GH release; and
Ghrelin which is a modulating hormone and in essence optimizes the balance between the "on" hormone & the "off" hormone. Before Ghrelin was discovered the synthetic growth hormone releasing peptides (GHRPs) were created and are superior to Ghrelin in that they do not share Ghrelin's lipogenic behavior. These GHRPs are GHRP-6, GHRP-2, Hexarelin and later Ipamorelin all of which behave in similar fashion.
In the aging adult these Ghrelin-mimetics or the GHRPs restore a more youthful ability to release GH from the pituitary as they turn down somatostatin's negative influence which becomes stronger as we age and turn up growth hormone releasing hormone's influence which becomes weaker as we age.
The exogenous administration of Growth Hormone Releasing Hormone (GHRH) creates a pulse of GH release which will be small if administered during a natural GH trough and higher if administered during a rising natural GH wave.
Growth Hormone Releasing Peptides (GHRP-6, GHRP-2, Hexarelin) are capable of creating a larger pulse of GH on their own then GHRH and they do this with much more consistency and predictability without regard to whether a natural wave or trough of GH is currently taking place.
Synergy of GHRH + GHRP
It is well documented and established that the concurrent administration of Growth Hormone Releasing Hormone (GHRH) and a Growth Hormone Releasing Peptide (GHRP-6, GHRP-2 or Hexarelin) results in synergistic release of GH from pituitary stores. In other words if GHRH contributes a GH amount quantified as the number 2 and GHRPs contributed a GH amount quantified as the number 4 the total GH release is not additive (i.e. 2 + 4 = 6). Rather the whole is greater than the sum of the parts such that 2 + 4 = 10.
While the GHRPs (GHRP-6, GHRP-2 and Hexarelin) come in only one half-life form and are capable of generating a GH pulse that lasts a couple of hours re-administration of a GHRP is required to effect additional pulses.
Growth Hormone Releasing Hormone (GHRH) however is currently available in several forms which vary only by their half-lives. Naturally occurring GHRH is either a 40 or 44 amino acid peptide with the bioactive portion residing in the first 29 amino acids. This shortened peptide identical in behavior and half-life to that of GHRH is called Growth Hormone Releasing Factor and is abbreviated as GRF(1-29).
GRF(1-29) is produced and sold as a drug called Sermorelin. It has a short-half life measured in minutes. If you prefer analogies think of this as a Testosterone Suspension (i.e. unestered).
To increase the stability and half-life of GRF(1-29) four amino acid changes where made to its structure. These changes increase the half-life beyond 30 minutes which is more than sufficient to exert a sustained effect which will maximize a GH pulse. This form is often called tetrasubstituted GRF(1-29) (or modified) and unfortunately & confusingly mislabeled as CJC-1295. If you prefer analogies think of this as a Testosterone Propionate (i.e. short-estered).
Note that some may also refer to this as CJC-1295 without the DAC (Drug Affinity Complex).
Frequent dosing of either the aforementioned modified GRF(1-29) or regular GRF(1-29) is required and as previously indicated works synergistically with a GHRP.
In an attempt to create a more convenient long-lasting GHRH, a compound known as CJC-1295 was created. This compound is identical to the aforementioned modified GRF(1-29) with the addition of the amino acid Lysine which links to a non-peptide molecule known as a "Drug Affinity Complex (DAC)". This complex allows GRF(1-29) to bind to albumin post-injection in plasma and extends its half-life to that of days. If you prefer analogies think of this as a Testosterone Cypionate (i.e. long-estered). However this is not accurate. CJC-1295 results in continual GH bleed. Although natural pulsation still occurs CJC-1295 does nothing to increase those pulses. Instead it raises base levels of GH and creates a more feminized pattern of release. This not desirable.
Modified GRF(1-29)however when combined with a GHRP brings about a substantial pulse which has desirable effects.
What follows on this first page of the thread is:
- A Basic Peptide Primer (which introduces the concept & structure of peptides)
- A Brief Summary of Dosing and Administration (for someone that wants to know the "how to use" straight away)
- A Brief Summary of Dosing and Administration (for someone that wants to know the "how to use" straight away)
If all of this is a bit unclear because a lot of new concepts are thrown at you one of my original very thorough articles is available:
Post #5 - Basic Guide: Growth Hormone Secretagogues
Post #6 - Basic Guide: Growth Hormone Secretagogues (part II)
Post #6 - Basic Guide: Growth Hormone Secretagogues (part II)
I have only one pet-peeve and that is when someone refers to synthetic growth hormone as "real" growth hormone. The GH that your body produces is as real as it gets. It is what grew you from a fetus to a boy (girl) and from a boy (girl) to a man (woman). - DatBtrue
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Basic Peptide Primer
Written by Datbtrue
What is a peptide?Written by Datbtrue
Peptides (proteins) are present in every living cell and possess a variety of biochemical activities. Some peptides are synthesized in the ribosomes of a cell by translation of mRNA (messenger RNA) into hormones and signaling molecules for example. Other peptides are assembled (rather then synthesized) and become enzymes with a vast variety of functions. Peptides also make up the structure of receptors which await binding of hormones & signaling molecules.
A peptide is a molecule created by joining two or more amino acids. In general if the number of amino acids is less than fifty, these molecules are called peptides, while larger sequences are referred to as proteins.
So peptides can be thought of as tiny proteins. They are merely strings of amino acids.
Raw Constituents of Peptides (Amino Acids)
Amino acids are small molecules made up of atoms. As part of their structure they posses a grouping of a Nitrogen (N) atom bonded to two Hydrogen (H) atoms. This is called an amino group and written as (NH2). In addition their structure is also made up of a second grouping of a Carbon (C) atom bonded to two Oxygen (O) and one Hydrogen atom. This group is called a carboxyl group and is written as (COOH).
Between these two groupings are atoms and bonds unique to each amino acid. In other words all amino acids possess the two groupings (amino & carboxyl) as end-points between which are sandwiched a unique set of atoms.
Amino Acids
Inside the human body there are twenty standard amino acids used by cells in peptide biosynthesis (i.e. the cellular creation of peptides from amino acids). Our genetic code specifies how to synthesize peptides and proteins from these amino acids.
Amino acids are classified into two groups: essential amino acids and nonessential amino acids.
An essential amino acid is an indispensable amino acid which cannot be made by the body and must be supplied by food. These include isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine. Another amino acid - histidine is considered semi-essential because the body does not always require dietary sources of it.
Nonessential amino acids are made by the body from the essential amino acids or the routine breakdown of proteins. The nonessential amino acids are arginine, alanine, asparagine, aspartic acid, cysteine, glutamine, glutamic acid, glycine, proline, serine, and tyrosine.
All twenty amino acids are equally important in maintaining a healthy body. They are the raw constituents of peptides and proteins.
The standard abbreviations for amino acids come in two forms: a one letter form and a three letter form. They are:
A - Ala - Alanine
C - Cys - Cysteine
D - Asp - Aspartic Acid
E - Glu - Glutamic Acid
F - Phe - Phenylalanine
G - Gly - Glycine
H - His - Histidine
I - Ile - Isoleucine
K - Lys - Lysine
L - Leu - Leucine
M - Met - Methionine
N - Asn - Asparagine
P - Pro - Proline
Q - Gln - Glutamine
R - Arg - Arginine
S - Ser - Serine
T - Thr - Threonine
V - Val - Valine
W - Trp - Tryptophan
Y - Tyr - Tyrosine
Amino acids exist in either D (dextro) or L (levo) form. Most of the amino acids found in nature (and all within human cells) are of the L-form. As a generality all amino acids except glycine have a mirror image of the L-form. This mirror image is called the D-form. It is common when referring to the L-form (naturally occurring form) to leave off the "L" designation whereas the "D" designation is always explicitly written.
D-amino acids are found naturally in bacterial cell walls and used in some synthetic peptides to make a peptide more stable or more resistant to degradation.
Amino Acid + Amino Acid = Peptide
The amino acids are joined together by what is known as a "peptide bond". A "peptide bond" is a linkage in which the nitrogen atom of one amino acid (from the amino group (NH2) binds to the carbon atom of another amino acid's carboxyl group (COOH).
During this binding process a molecule of water is released. This is called a condensation reaction.
The resulting CO-NH bond is called a peptide bond, and the resulting molecule is called an amide.
On the following image note that the COOH group gives up an Oxygen Hydrogen (OH) bond and the NH2 group gives up a Hydrogen (H). This forms H2O, which is a water molecule which is not part of the newly created peptide. NOTE: in the following image the C (carbon) symbol is missing as it is assumed so I indicate it with a blue square.
This reaction creating a peptide bond between two amino acids creates a peptide. We can call this peptide (made up of two amino acids) a dipeptide.
This process can be repeated using the twenty amino acids as raw material to create longer peptide chains. Sometimes peptide chains consisting of fifty to 100 amino acids are called polypeptides. Often a peptide chain beyond 100 amino acids is called a protein.
GHRP-6 is a peptide made up of just six amino acids. It's structure is often written as His-DTrp-Ala-Trp-DPhe-Lys-NH2
Note that the Carboxyl grouping (COOH) is assumed in the first position and is usually not written. The amino group (NH2) is wrtitten in the last position. The "meat" or the part that makes GHRP-6 distinct is the seqence in the middle of histadine bonded to the "D" form of Tryptophan bonded to Alanine bonded to Tryptophan bonded to the "D" form of Phenylalanine bonded to Lysine.
Pepdide bonds are formed by water (H2O) condensation (removing water). The converse is also true. A peptide bond can be broken down by hydrolysis (adding water).
The Amino Acid Structures of Peptides discussed in this thread
Growth Hormone Releasing peptides (GHRPs) (GH pulse initiators):
- GHRP-6 (His-DTrp-Ala-Trp-DPhe-Lys-NH2)
- GHRP-2 (DAla-D-2-Nal-Ala-Trp-DPhe-Lys-NH2)
- Hexarelin (His-D-2-methyl-Trp-Ala-Trp-DPhe-Lys-NH2)
- Ipamorelin (Aib-His-D-2-Nal-DPhe-Lys-NH2) - Ref-1
- GHRP-2 (DAla-D-2-Nal-Ala-Trp-DPhe-Lys-NH2)
- Hexarelin (His-D-2-methyl-Trp-Ala-Trp-DPhe-Lys-NH2)
- Ipamorelin (Aib-His-D-2-Nal-DPhe-Lys-NH2) - Ref-1
NOTES:
Aib = Aminoisobutyryc acid
D-2-Nal = "D" form of 2’-naphthylalanine
D-2-Nal = "D" form of 2’-naphthylalanine
Growth Hormone Releasing Hormone (GHRH) (amplifies the GHRP initiated pulse):
- Growth Hormone Releasing Hormone (GHRH) aka GRF(1-44) (Tyr-Ala-Asp-Ala-Ile-Phe-Thr-Asn-Ser-Tyr-Arg-Lys-Val-Leu-Gly-Gln-Leu-Ser-Ala-Arg-Lys-Leu-Leu-Gln-Asp-Ile-Met-Ser-Arg-Gln-Gln-Gly-Glu-Ser-Asn-Gln-Glu-Arg-Gly-Ala-Arg-Ala-Arg-Leu-NH2) = half-life "less then 10 minutes", perhaps as low as 5 minutes. - Ref-2
- GRF(1-29) aka Sermorelin (Tyr-Ala-Asp-Ala-Ile-Phe-Thr-Asn-Ser-Tyr-Arg-Lys-Val-Leu-Gly-Gln-Leu-Ser-Ala-Arg-Lys-Leu-Leu-Gln-Asp-Ile-Met-Ser-Arg-NH2) - the biologically active portion of the 44 amino acid GHRH = half-life "less then 10 minutes", perhaps as low as 5 minutes. - Ref-3
- GRF(1-29) aka Sermorelin (Tyr-Ala-Asp-Ala-Ile-Phe-Thr-Asn-Ser-Tyr-Arg-Lys-Val-Leu-Gly-Gln-Leu-Ser-Ala-Arg-Lys-Leu-Leu-Gln-Asp-Ile-Met-Ser-Arg-NH2) - the biologically active portion of the 44 amino acid GHRH = half-life "less then 10 minutes", perhaps as low as 5 minutes. - Ref-3
- Longer-lasting analogs of GRF(1-29):
-- replace the 2nd amino acid Alanine w/ D-Alanine only to modify GRF(1-29), D-Ala2 GRF(1-29) (Tyr-DAla-Asp-Ala-Ile-Phe-Thr-Asn-Ser-Tyr-Arg-Lys-Val-Leu-Gly-Gln-Leu-Ser-Ala-Arg-Lys-Leu-Leu-Gln-Asp-Ile-Met-Ser-Arg-NH2) = half-life "closer to 10 minutes" - Ref-4
-- replace the 2nd, 8th, 15th & 27th amino acids & get modified GRF(1-29) or CJC-1295 w/o the DAC (i.e. the part that will bind to albumin & make the half-life days) (Tyr-DAla-Asp-Ala-Ile-Phe-Thr-Gln-Ser-Tyr-Arg-Lys-Val-Leu-Ala-Gln-Leu-Ser-Ala-Arg-Lys-Leu-Leu-Gln-Asp-Ile-Leu-Ser-Arg-NH2) = Half-life at least 30 minutes or so - Ref-5
-- CJC-1295 (Tyr-DAla-Asp-Ala-Ile-Phe-Thr-Gln-Ser-Tyr-Arg-Lys-Val-Leu-Ala-Gln-Leu-Ser-Ala-Arg-Lys-Leu-Leu-Gln-Asp-Ile-Leu-Ser-Arg-Lys-(Maleimidopropionyl)-NH2) = Half-life measured in days, - Ref-6
-- replace the 2nd, 8th, 15th & 27th amino acids & get modified GRF(1-29) or CJC-1295 w/o the DAC (i.e. the part that will bind to albumin & make the half-life days) (Tyr-DAla-Asp-Ala-Ile-Phe-Thr-Gln-Ser-Tyr-Arg-Lys-Val-Leu-Ala-Gln-Leu-Ser-Ala-Arg-Lys-Leu-Leu-Gln-Asp-Ile-Leu-Ser-Arg-NH2) = Half-life at least 30 minutes or so - Ref-5
-- CJC-1295 (Tyr-DAla-Asp-Ala-Ile-Phe-Thr-Gln-Ser-Tyr-Arg-Lys-Val-Leu-Ala-Gln-Leu-Ser-Ala-Arg-Lys-Leu-Leu-Gln-Asp-Ile-Leu-Ser-Arg-Lys-(Maleimidopropionyl)-NH2) = Half-life measured in days, - Ref-6
NOTES:
Lys = linker to the Drug Affinity Complex (aka (Maleimidopropionyl))
"Since GH is released in a pulsatile manner and a higher level of GH is observed between 15 and 30 min after subcutaneous administration of GH-RH analogues, hydrolysis by trypsin-like enzymes could not affect the result of stimulation." - Potent Trypsin-resistant hGH-RH Analogues, JAN IZDEBSKI, J. Peptide Sci. 10: 524–529 (2004)
The analog in the above quoted study resisted degradation for 30 minutes. The quote implies that if your analog can last 30 minutes it has tapped out the potential for a single pulse.
Since another pulse won't be generated for about 2.5 - 3 hours analogs that last more than 30 minutes up to 3 hours are not any more beneficial.
You would need an analog that kept growth hormone releasing hormone around beyond 3 hours to have it trigger a second pulse.
Otherwise dosing the 30 minute analog every 3 hours will maximize GH output OR you could just use an analog such as CJC-1295 which lasts for many days and will trigger several GH pulses a day for several days on a single dose.
References:
Ref-1 - "lack of effect on ACTH and cortisol plasma levels" - Ipamorelin, the first selective growth hormone secretagogue , K Raun, European Journal of Endocrinology, 1996 Vol 139, Issue 5, 552-561
Ref-2 - Rapid enzymatic degradation of growth hormone-releasing hormone by plasma in vitro and in vivo to a biologically inactive product cleaved at the NH2 terminus, Frohman LA, J Clin Invest. 1986 78:906–913 and Incorporation of D-Ala2 in Growth Hormone-Releasing Hormone-( l-29)-NH2 Increases the Half-Life and Decreases Metabolic Clearance in Normal Men, STEVEN SOULE, Journal of Clinical Endocrinology and Metabolism 1994 Vol. 79, No. 4
Ref-3 - Rapid enzymatic degradation of growth hormone-releasing hormone by plasma in vitro and in vivo to a biologically inactive product cleaved at the NH2 terminus, Frohman LA, J Clin Invest. 1986 78:906–913 and Incorporation of D-Ala2 in Growth Hormone-Releasing Hormone-( l-29)-NH2 Increases the Half-Life and Decreases Metabolic Clearance in Normal Men, STEVEN SOULE, Journal of Clinical Endocrinology and Metabolism 1994 Vol. 79, No. 4
Ref-4 - Incorporation of D-Ala2 in Growth Hormone-Releasing Hormone-( l-29)-NH2 Increases the Half-Life and Decreases Metabolic Clearance in Normal Men, STEVEN SOULE, Journal of Clinical Endocrinology and Metabolism 1994 Vol. 79, No. 4
Ref-5 - See: Posts within this thread
Ref-6 - See: Posts within this thread
Ref-1 - "lack of effect on ACTH and cortisol plasma levels" - Ipamorelin, the first selective growth hormone secretagogue , K Raun, European Journal of Endocrinology, 1996 Vol 139, Issue 5, 552-561
Ref-2 - Rapid enzymatic degradation of growth hormone-releasing hormone by plasma in vitro and in vivo to a biologically inactive product cleaved at the NH2 terminus, Frohman LA, J Clin Invest. 1986 78:906–913 and Incorporation of D-Ala2 in Growth Hormone-Releasing Hormone-( l-29)-NH2 Increases the Half-Life and Decreases Metabolic Clearance in Normal Men, STEVEN SOULE, Journal of Clinical Endocrinology and Metabolism 1994 Vol. 79, No. 4
Ref-3 - Rapid enzymatic degradation of growth hormone-releasing hormone by plasma in vitro and in vivo to a biologically inactive product cleaved at the NH2 terminus, Frohman LA, J Clin Invest. 1986 78:906–913 and Incorporation of D-Ala2 in Growth Hormone-Releasing Hormone-( l-29)-NH2 Increases the Half-Life and Decreases Metabolic Clearance in Normal Men, STEVEN SOULE, Journal of Clinical Endocrinology and Metabolism 1994 Vol. 79, No. 4
Ref-4 - Incorporation of D-Ala2 in Growth Hormone-Releasing Hormone-( l-29)-NH2 Increases the Half-Life and Decreases Metabolic Clearance in Normal Men, STEVEN SOULE, Journal of Clinical Endocrinology and Metabolism 1994 Vol. 79, No. 4
Ref-5 - See: Posts within this thread
Ref-6 - See: Posts within this thread
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
A Brief Summary of Dosing and Administration
Dosing GHRPs
The saturation dose in most studies on the GHRPs (GHRP-6, GHRP-2, Ipamorelin & Hexarelin) is defined as either 100mcg or 1mcg/kg.
What that means is that 100mcg will saturate the receptors fully, but if you add another 100mcg to that dose only 50% of that portion will be effective. If you add an additional 100mcg to that dose only about 25% will be effective. Perhaps a final 100mcg might add a little something to GH release but that is it.
So 100mcg is the saturation dose and you could add more up to 300 to 400mcg and get a little more effect.
A 500mcg dose will not be more effective then a 400mcg, perhaps not even more effective then 300mcg.
The additional problems are desensitization & cortisol/prolactin side-effects.
Ipamorelin is about as efficacious as GHRP-6 in causing GH release but even at higher dose (above 100mcg) it does not create prolactin or cortisol.
GHRP-6 at the saturation dose 100mcg does not really increase prolactin & cortisol but may do so slightly at higher doses. This rise is still within the normal range.
GHRP-2 is a little more efficacious then GHRP-6 at causing GH release but at the saturation dose or higher may produce a slight to moderate increase in prolactin & cortisol. This rise is still within the normal range although doses of 200 - 400mcg might make it the high end of the normal range.
Hexarelin is the most efficacious of all of the GHRPs at causing an increase in GH release. However it has the highest potential to also increase cortisol & prolactin. This rise will occur even at the 100mcg saturation dose. This rise will reach the higher levels of what is defined as normal.
GHRP-6 at the saturation dose 100mcg does not really increase prolactin & cortisol but may do so slightly at higher doses. This rise is still within the normal range.
GHRP-2 is a little more efficacious then GHRP-6 at causing GH release but at the saturation dose or higher may produce a slight to moderate increase in prolactin & cortisol. This rise is still within the normal range although doses of 200 - 400mcg might make it the high end of the normal range.
Hexarelin is the most efficacious of all of the GHRPs at causing an increase in GH release. However it has the highest potential to also increase cortisol & prolactin. This rise will occur even at the 100mcg saturation dose. This rise will reach the higher levels of what is defined as normal.
Desensitization
GHRP-6 can be used at saturation dose (100mcg) three or four times a day without risk of desensitization.
GHRP-2 probably at saturation dose several times a day will not result in desensitization.
Hexarelin has been shown to bring about desensitization but in a long-term study the pituitary recovered its sensitivity so that there was not long-term loss of sensitivity at saturation dose. However dosing Hexarelin even at 100mcg three times a day will likely lead to some down regulation within 14 days.
If desensitization were to ever occur for any of these GHRPs simply stopping use for several days will remedy this effect.GHRP-2 probably at saturation dose several times a day will not result in desensitization.
Hexarelin has been shown to bring about desensitization but in a long-term study the pituitary recovered its sensitivity so that there was not long-term loss of sensitivity at saturation dose. However dosing Hexarelin even at 100mcg three times a day will likely lead to some down regulation within 14 days.
Chronic use of GHRP-6 at 100mcg dosed several times a day every day will not cause pituitary problems, nor significant prolactin or cortisol problems, nor desensitize.
GHRH
Now Sermorelin, GHRH (1-44) and GRF(1-29) all are basically GHRH and have a short half-life in plasma because of quick cleavage between the 2nd & 3rd amino acid. This is no worry naturally because this hormone is secreted from the hypothalamus and travels a short distance to the underlying anterior pituitary and is not really subject to enzymatic cleavage. The release from the hypothalamus and binding to somatotrophs (pituitary cells) happens quickly.
However when injected into the body it must circulate before finding its way to the pituitary and so within 3 minutes it is already being degraded.
That is why GHRH in the above forms must be dosed high to get an effect.
GHRH analogs
All GHRH analogs swap Alanine at the 2nd position for D-Alanine which makes the peptide resistant to quick cleavage at that position. This means analogs will be more effective when injected at smaller dosing.
The analog tetra or 4 substituted GRF(1-29) sometimes called CJC w/o the DAC or referred to by me as modified GRF(1-29) has other amino acid modifications. They are a glutamine (Gln or Q) at the 8-position, alanine (Ala or A) at the 15-position, and a leucine (Leu or L) at the 27-position.
The alanine at the 8th position enhances bioavailability but the other two amino substitutions are made to enhance the manufacturing process (i.e. create manufacturing stability).
For use in vivo, in humans, the GHRH analog known as CJC w/o the DAC or tetra (4) substituted GRF(1-29) or modified GRF(1-29) is a very effective peptide with a half-life probably 30+ minutes.
That is long enough to be completely effective.
The saturation dose is also defined as 100mcg.
Problem w/ Using any GHRH alone
The problem with using a GHRH even the stronger analogs is that they are only highly effective when somatostatin is low (the GH inhibiting hormone). So if you unluckily administer in a trough (or when a GH pulse is not naturally occurring) you will add very little GH release. If however you luckily administer during a rising wave or GH pulse (somatostatin will not be active at this point) you will add to GH release.
Solution is GHRP + GHRH analog
The solution is simple and highly effective. You administer a GHRH analog with a GHRP. The GHRP creates a pulse of GH. It does this through several mechanisms. One mechanism is the reduction of somatostatin release from the hypothalamus, another is a reduction of somatostatin influence at the pituitary, still another is increased release of GHRH from the brain and finally GHRPs act on the same pituitary cells (somatotrophs) as do GHRHs but use a different mechanism to increase cAMP formation which will further cause GH release from somatotroph stores.
GHRH also has a way of reciprocally reinforcing GHRPs action.
The result is a synergistic GH release.
The GH is not additive it is synergistic. By that I mean:
If GHRH by itself will cause a GH release valued at 2
and GHRP itself will cause a GH release valued at 5
Together the GH is not 7 (5+2) it turns out to say 16!
and GHRP itself will cause a GH release valued at 5
Together the GH is not 7 (5+2) it turns out to say 16!
A solid protocol
A solid protocol would be to use a GHRP + a GHRH analog pre-bed (to support the nightime pulse) and once or twice throughout the day.
For anti-aging, deep restful restorative sleep, the once at night dosing is all you need. For an adult aged 40+ it is enough to restore GH to youthful levels.
However for bodybuilding or fatloss or injury repair multiple dosings can be effective.
The GHRH analog can be used at 100mcg and as high as you want without problems.
The GHRP-6 can always be used at 100mcg w/o problems but a dose of 200mcg will probably be fine as well.
Again desensitization is something to keep an eye on particularly with the highest doses of GHRP-2 and all doses of Hexarelin.
So 100 - 200mcg of GHRP-6 + 100 - 500mcg+ of a GHRH analog taken together will be effective.
This may be dosed several times a day to be highly effective.
A solid approach is a bit more conservative at 100mcg of GHRP-6 + 100mcg of a GHRH analog dosed either once, twice, three or four times a day.
When dosing multiple times a day at least 3 hours should separate the administrations.
The difference is once a day dosing pre-bed will give a youthful restorative amount of GH while multiple dosing and or higher levels will give higher GH & IGF-1 levels when coupled with diet & exercise will lead to muscle gain & fatloss.
Dose w/o food
Administration should ideally be done on either an empty stomach or with only protein in the stomach. Fats & carbs blunt GH release. So administer the peptides and wait about 20 minutes (no more then 30 but no less then 15 minutes) to eat. AT that point the GH pulse has about hit the peak and you can eat what you want.
Last edited by a moderator: